crmPack
A new Crowd-Funding Project
October 6, 2025
Overview
New crowd-funding project
- Adaptive dose escalation trials are a key tool for the Phase 1 development both in oncology and non-oncology.
- The R package
crmPackhas been developed over the last 10 years to facilitate the design and analysis of these trials. - Until recently, while
crmPackhas a rich set of functionality and is actively used by several companies, several capabilities ofcrmPackare still missing and the package maintenance was not funded. - Therefore we launched a new crowd-funding model to ensure the sustainability, documentation and training of
crmPackand to develop new functionalities, which has the potential to makecrmPackthe new standard for adaptive dose escalation trials. - The model is along the lines of the
rpactfunding model, which has been successful
Acknowledgments to All Package Contributors
- Clara Beck (Bayer)
- Oliver Boix (Bayer)
- Prerana Chandratre (Bayer)
- Robert Adams (Bayer)
- Dimitris Kontos (ClinBAY/Bayer)
- Jiawen Zhu (Genentech)
- Ziwei Liao (Genentech)
- Daniel Sabanés Bové (Roche/RCONIS)
- John Kirkpatrick (Roche/Astellas)
- Giuseppe Palermo (Roche)
- Guanya Peng (Roche)
- Doug Kelkhoff (Roche)
- Wojciech Wojciak (Roche)
- Marlene Schulte-Goebel (Merck)
- Burak Kuersad Guenhan (Merck)
- Wai Yin Yeung (University of Lancaster/Roche)
- Thomas Jaki (University of Lancaster/Cambridge/Regensburg)
Adaptive Dose Escalation Trials
- Play a crucial role in the early stages of drug development
- Aim to determine the appropriate dosage for a new drug in humans
- Gradually increase the dosage in a stepwise manner
- Gather data on efficacy and safety of the drug
- Balance therapeutic effect and avoidance of excessive toxicity
- Conducted with a small overall sample size (~ 20-50 patients)
- Allow close monitoring and analysis of the drug’s performance
- Applied across various therapeutic areas (i.e. not restricted to oncology)
- Examples:
- Rule-based designs: 3+3
- Model-assisted designs: Bayesian Optimal Interval Design (BOIN)
- Model-based designs: Continual Reassessment Method (CRM)
Illustration
Illustration
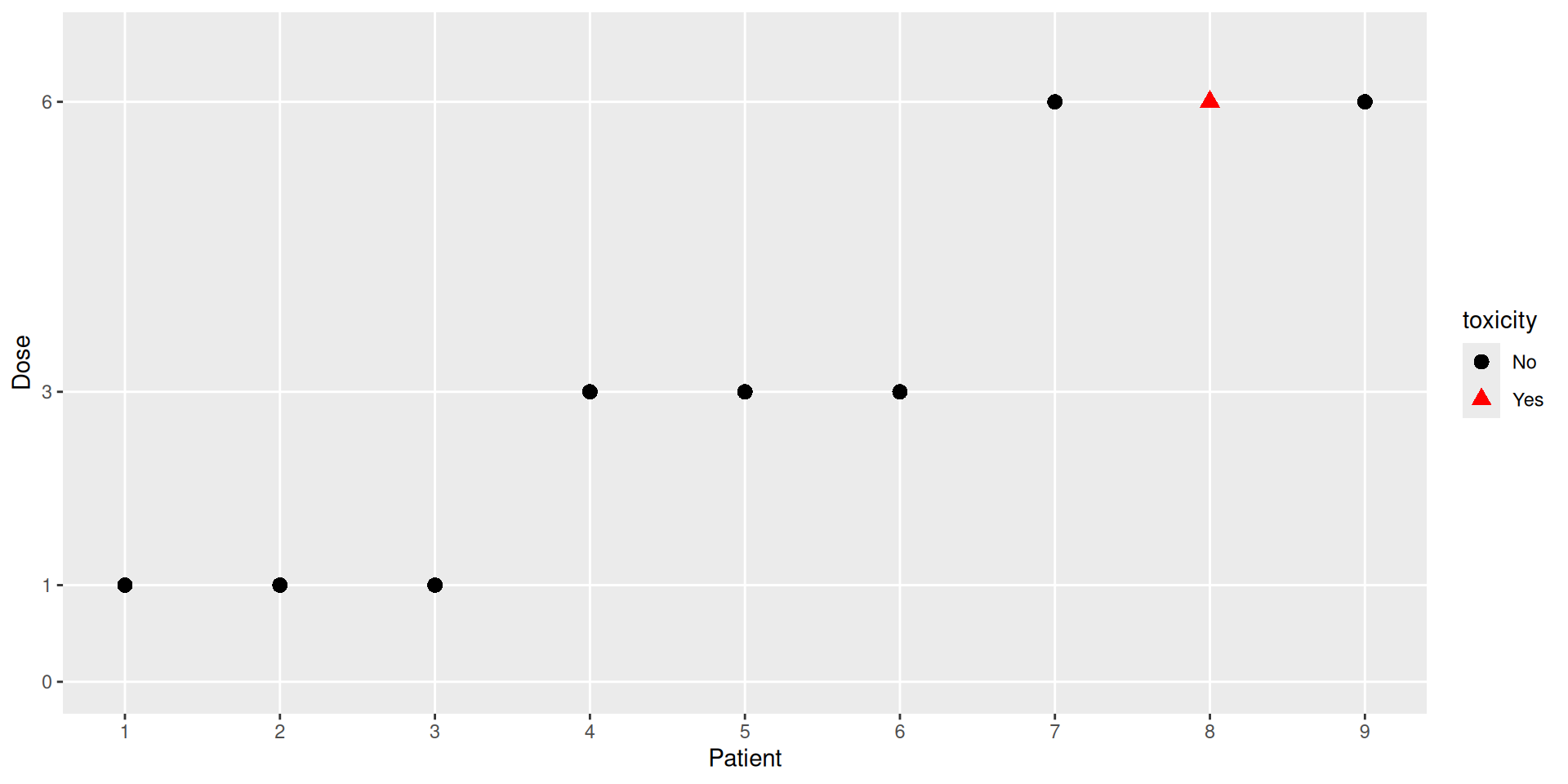
The R package crmPack
- Specialized R package for dose escalation trials
- Initial CRAN release in 2016, making it widely accessible to the R community
- Higher flexibility compared to other software, thanks to it modular design principles (S4 classes)
- Easy extensibility and adoption of new designs
- Produces visual and numeric output, enabling clear and intuitive presentation of trial results
- Facilitates simulations, allowing for the evaluation of various scenarios and assess the performance of different dose escalation strategies
crmPack Framework
crmPack provides a highly flexible framework for the design and analysis of dose escalation trials. This schematic illustrates the framework’s key components and their interactions:
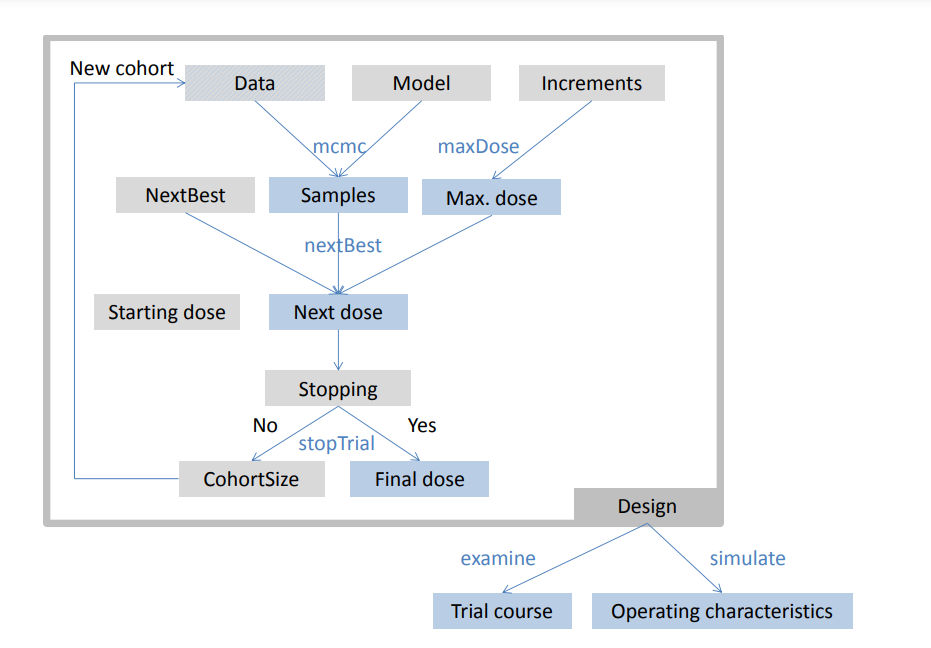
crmPack Framework
Development History
- Development started at Roche in 2014 (Daniel Sabanés Bové)
- Included a collaboration with Prof. Thomas Jaki, Wai Yin Yeung (University of Lancaster)
- Open-sourced in 2016 (link)
- Paper published in 2019 (link)
- Open source team started collaborating in 2021 (Bayer, Merck, Roche, Cambridge University) (slides)
- Daniel left Roche mid 2024
Other Software
- Proprietary software (e.g. FACTS, East Horizon)
- Websites (e.g. TrialDesign.org, MD Anderson Biostatistics Software)
- Open source R packages
- Model-based, i.e. CRM designs:
bcrm,blrm,dfcrm,OncoBayes2, … - Model-assisted, e.g.
BOIN - Wrapper packages, e.g.
escalation
- Model-based, i.e. CRM designs:
- Advantages of
crmPack:- Combines model based and rule based designs in one package
- More flexible and extensible
- users can easily define models, designs, increments and stopping rules etc.
Download Statistics
Median daily downloads of crmPack from CRAN each month since 2016 (new CRAN versions marked by red lines, last new features on CRAN before 2020)
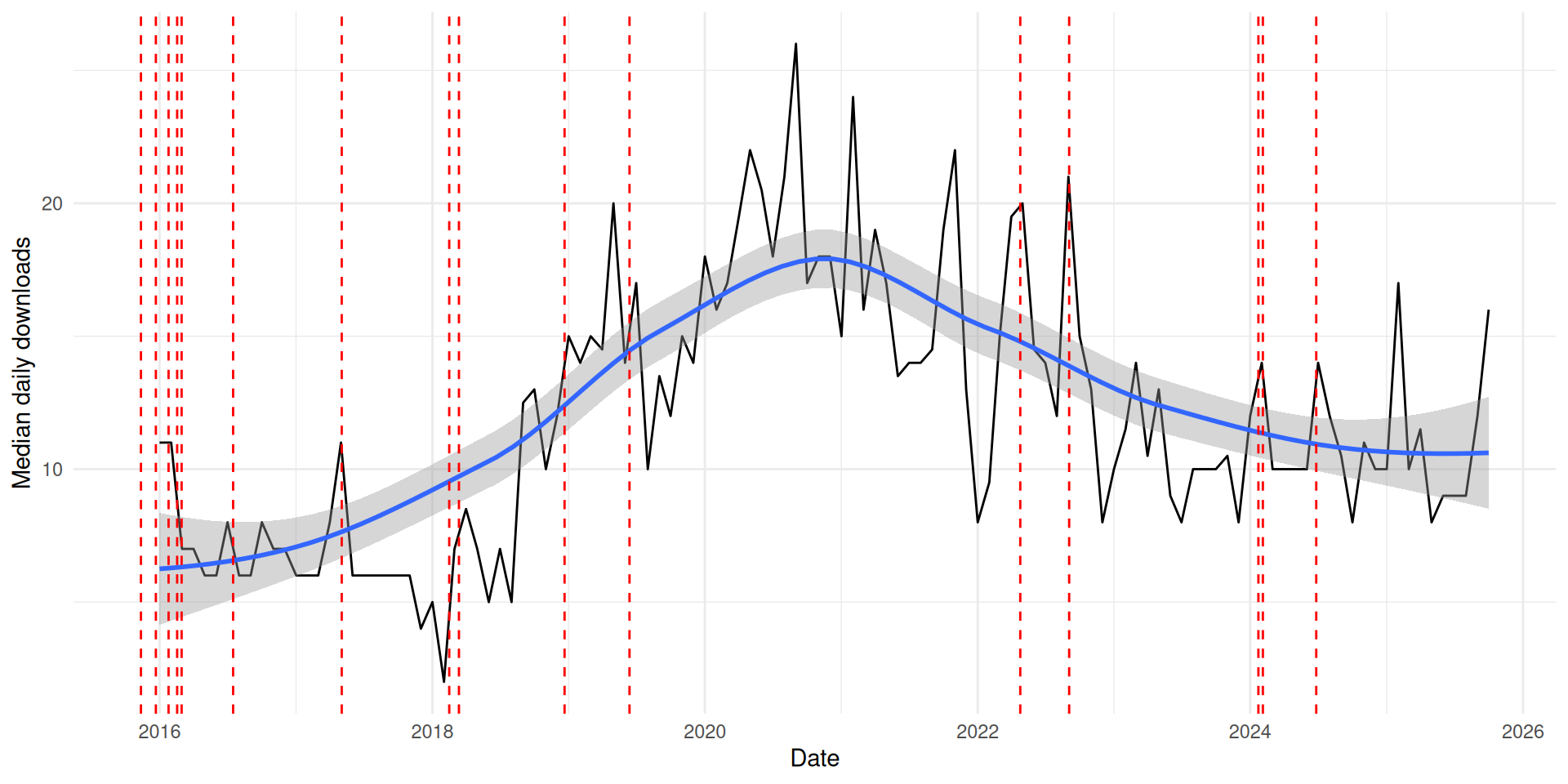
Monthly medians of daily downloads of crmPack from CRAN (obtained via cranlogs)
Testimonials
Burak and Marlene (Merck):
- “We already used and continuously use successfully for clinical trials in regulatory context, and we appreciate the flexibility that you can adapt it to your needs”
- “As we were part of development team, we are aware that development version has a high software quality”
- “We have very positive experience collaborating and working with the lead developer (Daniel) over the years”
Testimonials (cont’d)
Dimitris and Oliver (Bayer):
- “Implementation of a dose escalation study can be achieved without requiring in-depth knowledge of R programming.”
- “Comprehensive supporting documentation, available vignettes and well structured code base enables easy implementation of new design features.”
- “Collaboration with the
crmPackdevelopment team has been rewarding over the past few years.”
Testimonials (cont’d)
Uli (Roche):
“We started to use CRM dose escalation designs in early development oncology and non-oncology more than 10 years ago. At the beginning crmPack was not yet available and especially the simulation part was a little cumbersome. In the meantime, crmPack is our standard package used for CRM dose-escalation methods. Based on crmPack we also developed templates for the protocol sections and appendix, which includes simulations for different assumed dose-toxicity relationships. With the help of crmPack a model based dose escalation design is no longer a burden also for new-comers and colleagues who have not worked in early development before.”
Testimonials (cont’d)
Anonymous (CRO):
“Running a CRM trial rather than a 3+3 saved us a year in development time and almost USD 1M as well as providing greater insight to the relationship between dose and toxicity”
Current Status
The current development version of crmPack has the following features:
- Binary, time-to-event, dual efficacy/safety, ordinal outcomes
- 15 different models
- 14 different recommendation options
- 19 different stopping rules
- 9 cohort size rules
- 9 increment rules
- Reporting functionality (using
knitr) - 9 vignettes (documentation)
Collaborative Funding Model
- Similar as for
rpactvia a Service Level Agreement withRPACT- Short contract (couple of pages) which is simple for procurement
- No new vendors needed
- Friedrich and Gernot also contribute as developers as appropriate
- Use of existing validation infrastructure
- 4 pharma companies already committed/signed the agreement
- Current service level fee of 17,000 EUR per year per company
Service Level Agreement Benefits
- Benefits include:
- Maintenance of the package with regular updates
- Support for the package with any questions/issues
- Development of new open source features (if not too large)
- Yearly training of users in the company
- Formal GxP validation documentation
- Installation Qualification on company servers
- Options: in-house graphical user interfaces, automation support, private (not open source published) extensions, customer specific programs
